A student in the group next to you is not following the safety rules. A hydrogen atoms form an ionic bond with another atom on an adjacent molecule.

What Is A Hydrogen Bond Hydrogen Bond Examples Video Lesson Transcript Study Com
Water is an excellent example of hydrogen bonding.
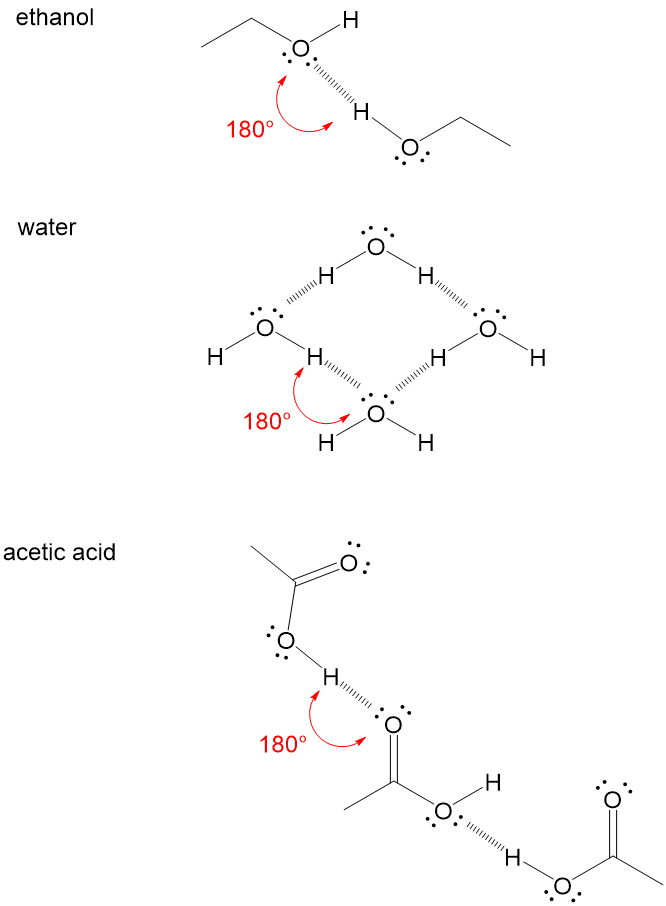
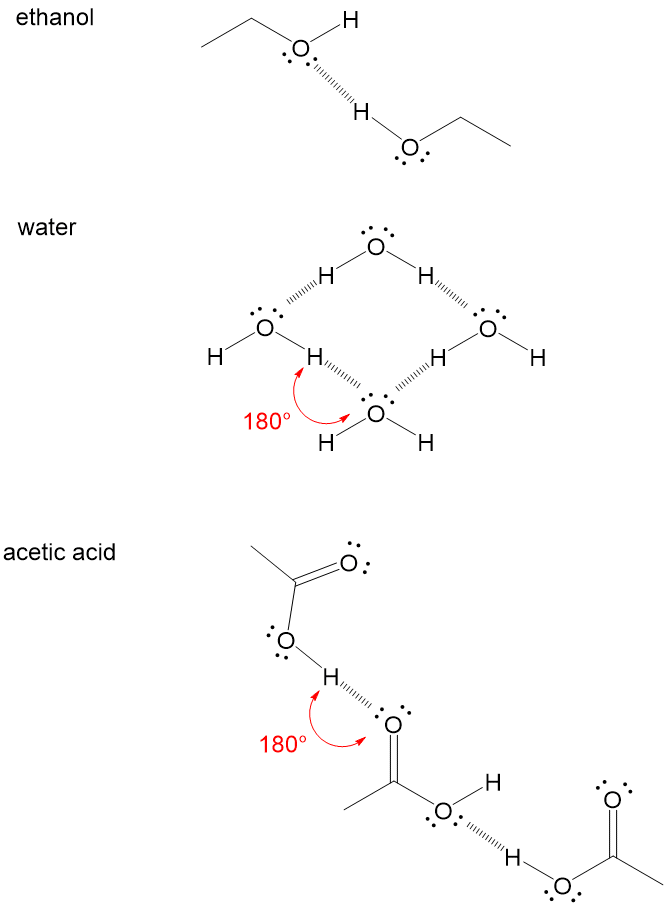
. So lets take this first one and sketch it out a little bit so we can see whats bonded to that. A dashed line is used to represent a possible hydrogen bond Note. This is a special type of hydrogen bond where the proton is usually placed in the middle between two identical atoms.
He manages to spill a large amount of solution on his clothes and THEN. For which of the following is hydrogen bonding NOT a factor. The symmetric hydrogen bond is a type of a three-centre four-electron bond.
These attractions can occur between molecules inter. The strength of a typical hydrogen bond is about 5. Hydrogen bonding refer to am electrostatic force of attraction that exist between a hydrogen atom which is covalently bonded together to an atom that is more electronegative and another electronegative atom that have a lone pair of.
Which of the following pure compounds will exhibit hydrogen bonding. A hydrogen bond is the electromagnetic attraction created between a partially positively charged hydrogen atom attached to a highly electronegative atom and another nearby electronegative atom. How this works is that the polar nature of the water molecule means each.
A dashed line is used to represent a possible hydrogen bond. A hydrogen bond tends to be stronger than van der Waals forces. Water molecules completely separate into ions in solutions.
A hydrogen bond is a type of dipole-dipole interaction. A hydrogen atom in a molecule forms a bond with any atom. Which of the following is a direct result of hydrogen bonding.
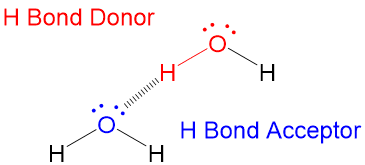
Hydrogen bond is when you have a hydrogen bonded directly to a nitrogen oxygen or flooring And the nitrogen oxygen or Florian has to have at least one lone pair. Jhumajana482 jhumajana482 24092019. A hydrogen atom bonded to F O or N is attracted.
Video Explanation Solve any question of Chemical Bonding and Molecular Structure with-. It is not a true chemical bond. Because oxygen is more electronegative than hydrogen the electrons in a water molecule spend more time closer to _______.
A mixture of copper and zinc was dropped in a flask containing. The bond is between the hydrogen of one water molecule and the oxygen atoms of another water molecule not between the two hydrogen atoms a common misconception. Oh So the CH three groups and then theres an oh and then theres an H.
It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a N O or F atom and another very electronegative atom. Which of the following pure compounds will exhibit hydrogen bonding. R C H 2 N H C H 3 shows the hydrogen bonding because H-atom is directly attahced to N-atom.
Water expands and becomes less dense when it freezes. A hydrogen atom forms a covalent bond with another atom. Get solutions Get solutions Get solutions done loading Looking for the textbook.
The strength of the bond between each atom is equal. This is due to unequal electronegativity causing increased electron density for the more electronegative atom and therefore a more. Examples of Hydrogen Bonds.
Solutions for Chapter 9 Problem 30QP. SiH2 show the same structure as water but not exhibit hydrogen bonding -the difference in their melting and boiling points illustrate the effect hydrogen bonding has eg. CH3OCH3 C₂H4 NH3 CH3CH2OH CH20 C₂H₂ CH3NH2 C3Hs.
Hydrogen bond strengths range from 4 kJ to 50 kJ. CH3OCH3 C₂H4 NH3 CH3CH2OH CH20 C₂H₂ CH3NH2 C3Hs. Water H 2 O.
Water absorbs heat when it evaporates and forms a gas. MnO4 aq Fe2- aq Mn2 aq Fe3 aq. Up to 10 cash back Hydrogen bonding occurs when a molecule contains a hydrogen atom bonded to fluorine oxygen or nitrogen.
This bond is also much stronger compared to the normal hydrogen bond. A hydrogen bond is a type of attractive dipole-dipole interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom. This hydrogen becomes partially positive in charge while the attached atom becomes partially negative.
Which of the following molecule does not show hydrogen bondingaHFbH2OcHIdH2S Get the answers you need now. Which of the following compounds show hydrogen bondinga CH3Fb HOOHc NH3Fd H3COCH3. Question 29 x Incorrect.
A water molecule consists of one oxygen atom joined to each of two hydrogen atoms by a n _______ a type of bond in which the electrons do not spend equal time with the two atoms involved. A hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded to a small highly electronegative atom is attracted to a lone pair of electrons on an atom in a neighboring molecule. Hydrogen bonding is a special type of dipole-dipole attraction between molecules not a covalent bond to a hydrogen atom.
The molecules that exhibit hydrogen bonding are ammonia NH3 acetic acid CH3CO2H and H2C20H. Other hydrides of group 16 elements eg. H2O boils at 100C whereas H2S boils at roughly -60C.
What is hydrogen bonding. Question 29 x Incorrect. Hydrogen bonds are very strong compared to other dipole interactions.
Hydrogen bonding occurs in molecules when ___________________. Movement by osmosis the polar ends of a water molecule the bent structure of a water molecule cohesion and adhesion. This bond always involves a hydrogen atom.
Hydrogen bonds can occur between molecules or within parts of a single molecule. Balance the following redox reaction in acidic medium. Water absorbs heat when it evaporates and forms a gas.
Water forms hydrogen bonds with ions and other polar substances. Which of the following properly shows the hydrogen bonding between amides.

Hydrogen Bonds In Water Article Khan Academy

How Do You To Tell When A Hydrogen Bond Will Occur
0 Comments